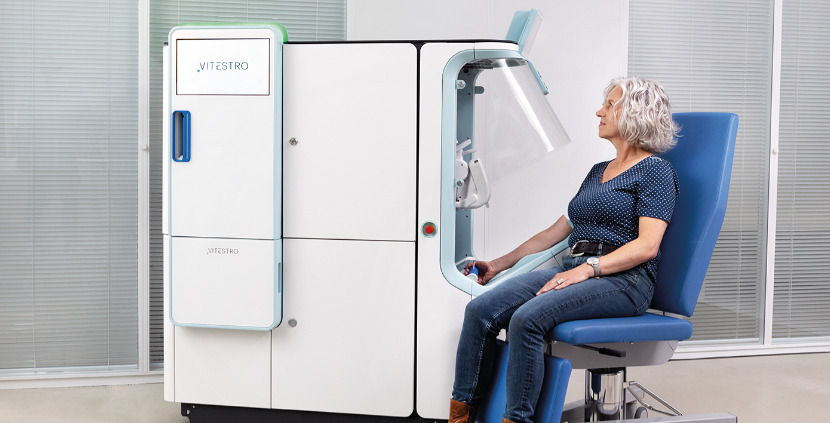
In an era where healthcare is increasingly intertwined with cutting-edge technology, Vitestro, a Dutch medical robotics company based in Utrecht, is pushing boundaries with Aletta – the world's first fully autonomous robotic phlebotomy device. Designed to automate the entire blood collection process, Aletta represents a leap forward in addressing chronic staffing shortages in medical facilities while enhancing patient comfort and procedural accuracy.
The Technology Behind Aletta
Aletta employs a sophisticated blend of artificial intelligence, multimodal imaging, and precision robotics to perform blood draws without human intervention. The device starts by applying a tourniquet, then uses near-infrared scanning and ultrasound to locate veins with submillimeter precision. Doppler ultrasound confirms the vein selection before the robot inserts the needle, collects samples into tubes (which it automatically changes and inverts), and finally applies a bandage – all while keeping the needle hidden from the patient's view to minimize anxiety.
This automation isn't just about speed; it's engineered for consistency. Traditional manual blood draws can vary based on the phlebotomist's experience, leading to success rates as low as 80-89% in challenging cases. Aletta, however, boasts a first-stick success rate of 95% based on interim results from the A.D.O.P.T. (Autonomous Blood Drawing Optimization and Performance Testing) trial, which involved over 4,000 patients across multiple European sites. Additionally, it achieves a remarkably low hemolysis rate of 0.6%, well below the 2% industry benchmark, ensuring higher-quality samples for lab analysis.
Tackling Healthcare Challenges
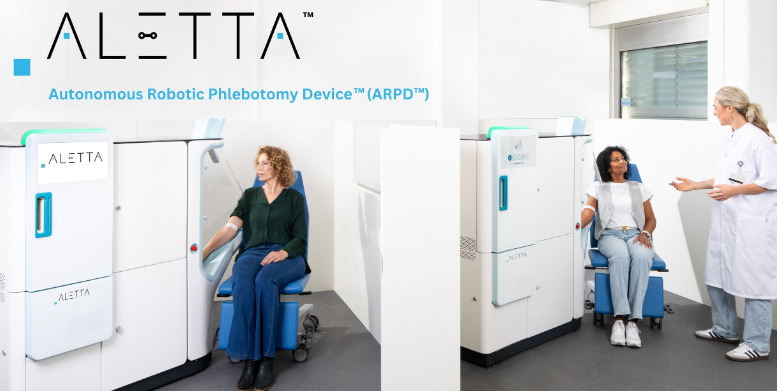 One of the primary drivers behind Aletta's development is the global shortage of skilled phlebotomists, exacerbated by rising demand for diagnostic testing. By automating routine procedures, the device allows one supervisor to oversee up to three units simultaneously, boosting efficiency in high-volume settings like hospitals and labs. This not only reduces wait times but also frees up human staff for more complex tasks.
One of the primary drivers behind Aletta's development is the global shortage of skilled phlebotomists, exacerbated by rising demand for diagnostic testing. By automating routine procedures, the device allows one supervisor to oversee up to three units simultaneously, boosting efficiency in high-volume settings like hospitals and labs. This not only reduces wait times but also frees up human staff for more complex tasks.
Patient experience is another key focus. In clinical trials, 85% of participants reported pain levels comparable to or less than manual draws, and 98% accepted the robotic procedure. The hidden needle and streamlined process – with a median duration of about 1 minute 49 seconds – help alleviate the stress often associated with blood tests, particularly for those with needle phobias or difficult veins. Adverse events were minimal, with only 1.3% mild cases and no moderate or serious incidents recorded.
Regulatory Milestones and Global Expansion as of 2026
By early 2026, Aletta has already made significant strides. It secured CE marking under the European Medical Device Regulation (MDR) in 2024, making it the first device of its kind approved for commercial use in the EU. It's now in routine clinical use at several Dutch hospitals, including OLVG Lab in Amsterdam, St. Antonius in Utrecht, and Result Laboratorium in Dordrecht, as part of ongoing trials.
In the United States, Vitestro is advancing toward FDA approval. As of January 2026, the company is conducting multi-center clinical studies with partners like Baylor Scott & White Research Institute, Northwestern Medicine, Advocate Health, and Intermountain Health. While not yet cleared by the FDA, Vitestro anticipates submitting its application in the latter half of 2025 or early 2026, with U.S. trials set to commence in 2027. The company recently launched a new website and released its first public video demonstration in January 2026, showcasing Aletta in action to build transparency and confidence among healthcare providers and patients.
Also read:
- The AI Coding Surge: Redefining Productivity, Organizations, and the Future of Work
- The Simplest AI Prompt Hack: Repeat Yourself for Smarter Responses
- Thriving in the AI Era: The Rise of Artisans and Automators Amid Mediocrity's Fall
Looking Ahead: A New Standard in Diagnostics
Vitestro's journey with Aletta underscores a broader trend toward automation in healthcare, aligning with fully automated lab workflows to improve preanalytical quality. Founded with a vision to standardize blood collection, the company has raised substantial funding and formed strategic partnerships to support global rollout. As Toon Overbeeke, Vitestro's CEO, emphasized in a recent release, this technology is about "enabling informed adoption" and establishing a responsible new standard.
With over 6,000 patient draws completed in Europe by mid-2025 and expanding trials, Aletta is poised to transform phlebotomy worldwide. In a future where robots handle routine tasks, human expertise can focus on care that truly requires a personal touch – making healthcare more efficient, accessible, and patient-centered.